Increasing operational efficiency for conducting clinical trial visits
Background
Science 37 is a research and technology company that specializes in decentralized clinical trials. The leadership team requested guidance on how to increase operational efficiency of our internal staff members that conduct clinical trial visits. To learn more, I created a research initiative to learn from the Clinical Research Coordinators (CRCs), Nurses, and Investigators who participate.
Problem
Research revealed that staff workflows for conducting visits were repetitive, manual, and tedious. Our product and operational teams wanted a read out to understand where these pinpoints occurred to inform how we:
Enhance visit operational efficiency
Alleviate cognitive burden
Minimize the risk of errors
Ultimately offer an enhanced experience for study participants
The research revealed areas of opportunity across internal processes, technology set up, and the Science 37 platform. These user-focused insights informed internal teams and product road maps.
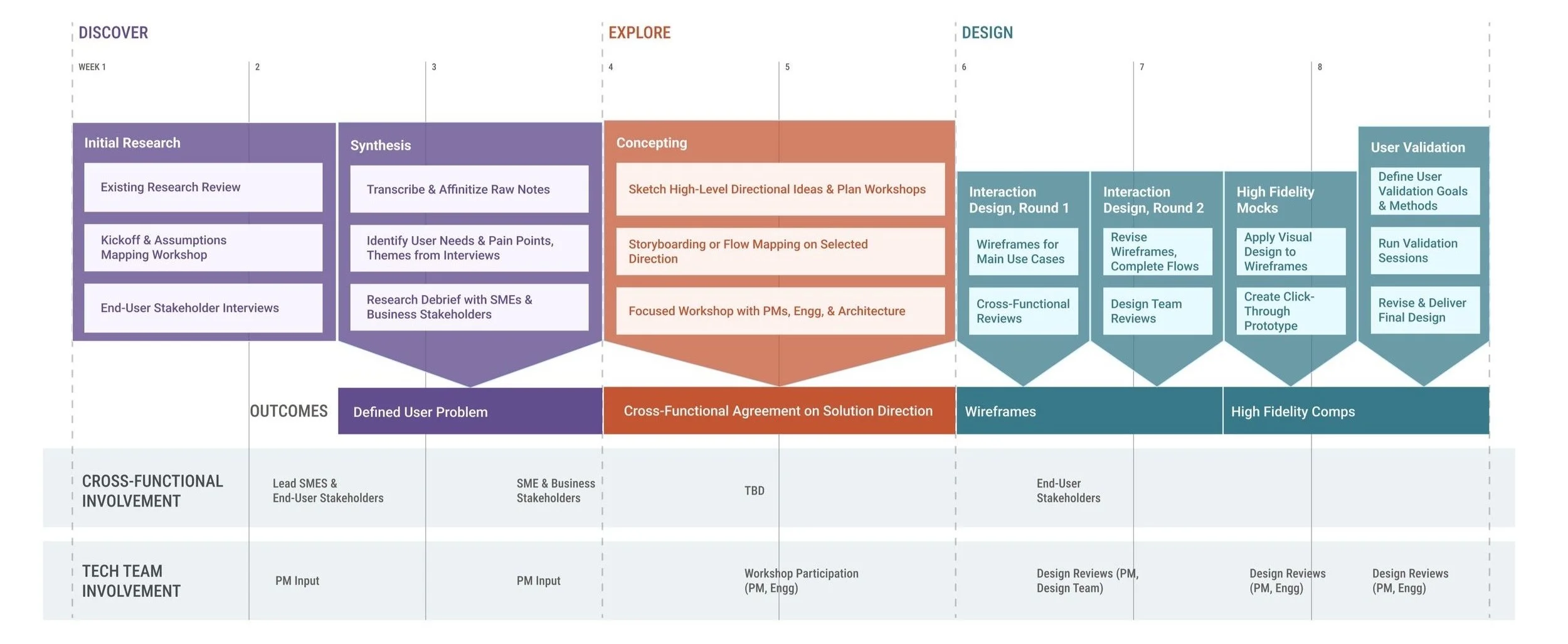
Research project plan
Conducting research
We wanted to hear from users who are on the ground running visits, whether that was virtually or within a participant’s home. We based our interview questions on the following research objectives:
Understand the set of stakeholders who will most benefit from support in managing visit.
Uncover opportunities to unblock study staff when completing a task.
Uncover opportunities to help study staff work more efficiently and reduce potential errors.
Facilitate a shared vision amongst business stakeholders and gain alignment on next steps.
Breaking down collected data by high-level categories gave us an overhead view of where the most activity was occurring by role throughout a visit
Synthesizing our Findings
After collecting raw notes from Clinical Research Coordinators (CRCs), Nurses, and Investigators, we grouped notes by topic. We quickly realized the Nurse experience was highly complex and required support from CRCs and Investigators to ensure they could complete visit tasks. We decided to make the Nurse experience our primary focus because of the secondary impact this would have on other roles.
To better understand the Nurse journey, I created a visualization to understand what was occurring before, during, and after a visit. This journey highlights the Nurse’s goals, concerns, touchpoint and actions, impact on other site staff members, as well as opportunities in both the platform and higher-level processes.
Final Recommendations
Improve Visit Navigation
How might we ensure study staff can find the most relevant visit and forms in the correct order?
Quick Access to Reference Materials
How might we integrate the most up-to-date protocol and visit guides within our platform?
Clear Participant Overview
How might we give a clear picture of participant status and give insight into special requests or concerns regarding their home environment?
Single-button Automation
How might we automate multi-step tasks that occur outside of the platform?
Scheduling Improvements
How might we provide a buffer for visit set up to avoid interference with time-sensitive visit activities?
These areas of opportunity provide the most impact in ensuring the Nurse visit experience improves, as well as reducing support workload for Clinical Research Coordinators and Investigators.